What is the difference between diffusion and osmosis?
Both diffusion and osmosis are two vital passive transport that facilitates the movement of molecules in and out of the cell.
The lesson provides the core difference between diffusion and osmosis in table form for easier understanding during revision for competitive exams.
What Is Diffusion?
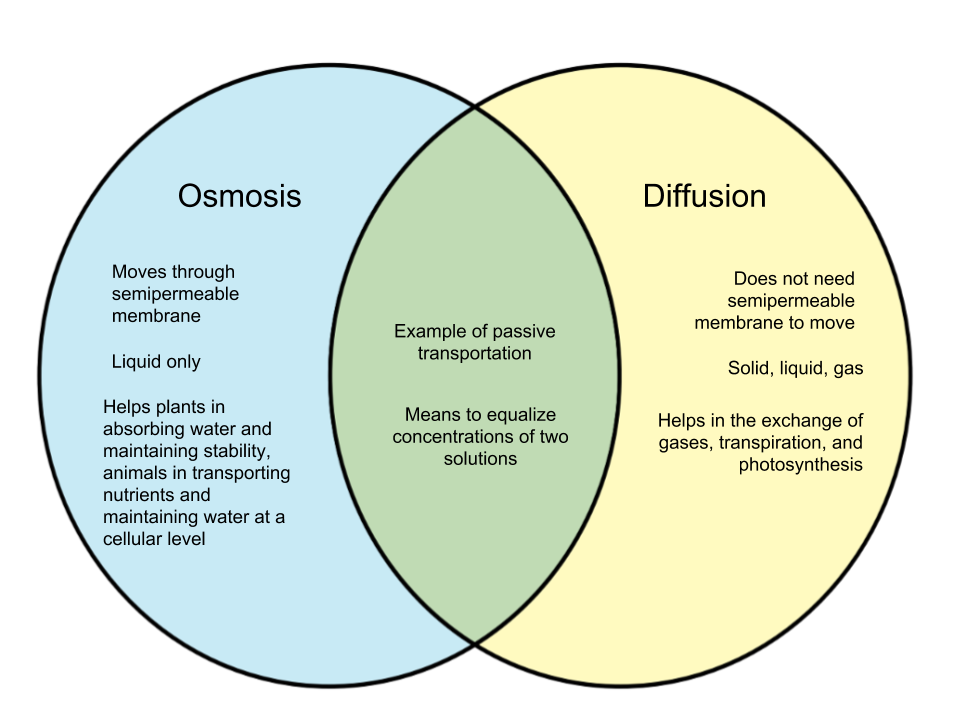
Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration.
Diffusion is quite important during the exchange of gases, transpiration, and photosynthesis in plants. It also helps in the creation of energy.
Types of diffusion include surface, collective, electron, facilitated, effusion, photon, gaseous, self, reverse, momentum, Brownian, and Knudsen diffusion.
Factors affecting diffusion are molecular weight, concentration gradient, pressure, and temperature.
Examples of situations entail diffusion is any spray such as deodorants and perfumes where the molecules of the gas distribute equally in the available space on the bottle is opened.
What Is Osmosis?
Osmosis is the movement of solvent molecules through a semi-permeable membrane from a dilute solution to a concentrated solution to attain equilibrium.
Osmosis help to maintain concentration gradient inside and outside the cell, distribution of nutrients, and release of the metabolic waste from the body.
Some common types of osmosis are reverse and forward osmosis. Factors that affect osmosis are diffusion distance, concentration gradient, and temperature.
Some of the common terms used during osmosis are the hypotonic solution, hypertonic solution, and isotonic solution.
Areas, where osmosis is applicable, are transfusion, edema, fluid balance, and blood volume as well as red blood cells and fragility.
Comparison Chart: Diffusion Vs Osmosis
| Basic Terms | Diffusion | Osmosis |
| Meaning | Movement of particles from a region of high concentration to a region of low concentration | Movement of solvent molecules through a semi-permeable membrane from dilute solution to concentrated solution |
| Example | Diffusion of ink in water | Plasmolysis of cell in a salt solution |
| Membrane | No membrane required | Semi-permeable membrane |
| Medium of occurrence | Gas to gas, liquid to gas, solid to gas and solid to liquid | Only in liquid |
| Substances | Solvent and solutes | Only solvent molecules |
| Types of solvent | Similar or dissimilar | Similar types only |
| Influenced by | Not by solute potential | By solution potential |
| Energy | Depend on free energy | Depend on energy reduction of one solvent |
| End result | Equilibrium attained | No equilibrium attained |
| Rate of process | Faster | Slower |
Core Difference Between Diffusion and Osmosis In Point Form
- Osmosis is limited to liquid medium while diffusion occurs in all mediums
- Osmosis requires a semi-permeable membrane while diffusion does not.
- Diffusion is the movement of particles from a region of high concentration to low concentration while osmosis the movement of water molecules via a semi-permeable membrane from dilute to the concentrated solution.
- Water molecules are the diffusing molecules in osmosis while diffusion can be solid, gases and liquid.
- Diffusion is a faster process while osmosis is a slower process.
Core Similarities Between Diffusion and Osmosis
- Both are types of passive transport
- Both are affected by certain factors
You May Also Like:
- Difference between Turgidity and Flaccidity
- Difference between Transpiration and Guttation
- Difference between Endosmosis and Exosmosis
- Difference between Plasmolysis and Deplasmolysis
- Difference between Hypertonic and Hypotonic Solutions
Comparison Video
Conclusion
These two passive transport are quite important in animals and plants. It is worth reading to get detailed insight into these terms.
The core difference between diffusion and osmosis is based on their definition as well as the presence of the semi-permeable membrane.
More Sources and References
- Osmosis. Wikipedia
- Diffusion and Osmosis. Khan Academy