What is the difference between Tyndall effect and Brownian motion?
The behavior of particles in a substance is described by the Tyndall effect and Brownian motion in Chemistry. Tyndall effect can be observed by passing light beams and Brownian motion through a light microscope.
The main difference between the Tyndall effect and Brownian motion is that the former occurs due to the scattering of light by individual particles while the latter due to the random motion of atoms or molecules in a fluid.
You May Also Enjoy: Difference between Mixture and Compound
Comparison Table (Tyndall Effect vs Brownian Motion)
| Basic Terms | Tyndall Effect | Brownian Motion |
| Meaning | It occurs due to the scattering of light as light beams pass through a colloidal solution | It is a random movement of particles in a fluid due to their collision with other atoms or molecules |
| Observation Medium | Human Eye | Light Microscope |
| Discovered by | John Tyndall | Botanist Robert Brown |
| Property | Optical property | Kinetic property |
| Concept | Light scattering phenomenon | The random movement of particles by collision |
| Applicable medium | Colloidal | Liquid and gases |
| Factors Affecting | Wavelengths of light, the density of a colloidal substance, frequency of light | Size of particles, temperature, viscosity, the concentration of particles |
| Size of the Substance | From 40 to 900nm | Smaller diameter |
| Examples | Milk solution, Soap solutions, Opalescent glass | Diffusion of dust, gases in the air, Diffusion of calcium to bones |
What Is Tyndall Effect?
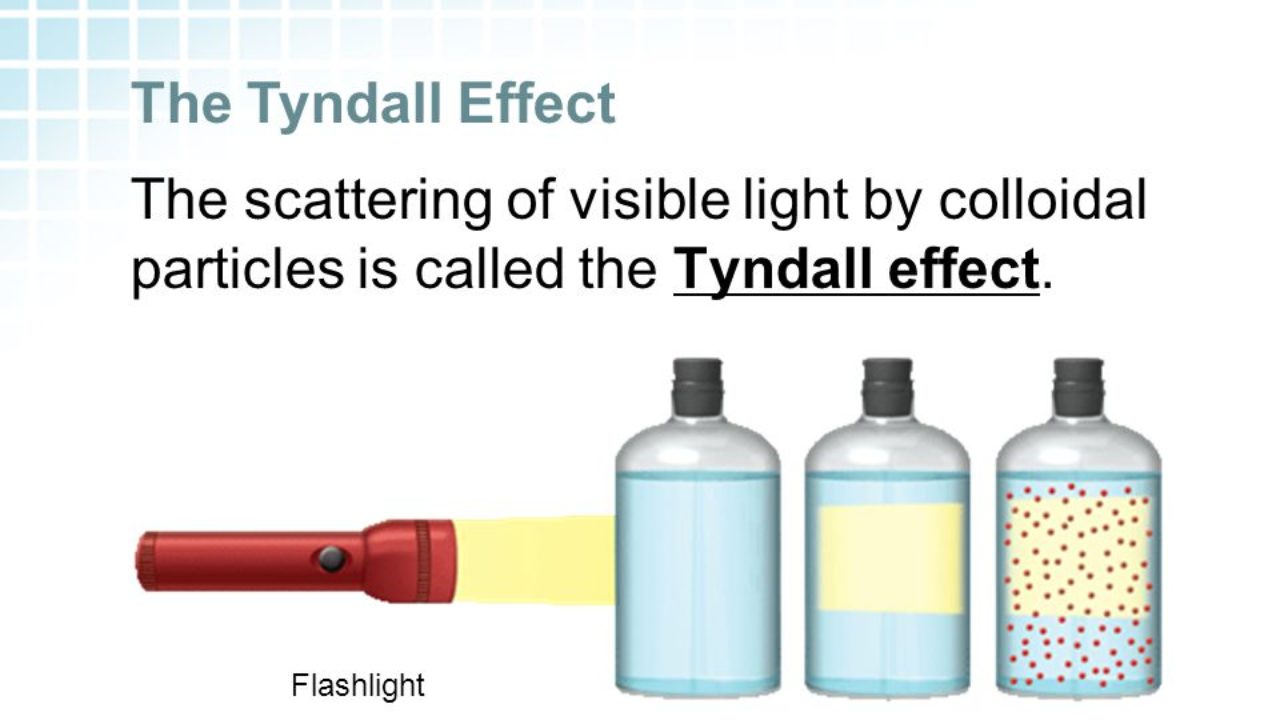
Tyndall effect occurs in colloidal fluids. Light gets scattered due to the presence of colloidal particles in the fluid. The scattering of light can be observed with naked eyes.
But the effect cannot be seen in a true solution. A true solution is a homogenous mixture of two or more substances. The suspension is a heterogeneous mixture containing different substances. Colloids are an intermediate between suspension and true solutions.
The Tyndall effect was discovered by John Tyndall. It provides the easiest way to distinguish between true and colloidal solutions.
Examples of Tyndall effects are the Blue eye color. Factors affecting the Tyndall effect are the frequency of the incident light beam and the density of particles.
What Is Brownian Motion?
Brownian motion is the random movement of particles in a fluid due to collision with other atoms or molecules. The Brownian motion makes the particles appear suspended in the fluid.
The experiment was first discovered by Botanist Robert Brown. Diffusion describes the movement of particles from the higher region to the lower region. But the random movement can be observed under a light microscope.
An example of Brownian motion is the motion of pollen grains on still water. Factors affecting Brownian motion are temperature and concentration.
You May Also Enjoy: Difference between Light Microscope and Electron Microscope
Main Difference between Tyndall Effect and Brownian Motion
- Tyndall effect is a phenomenon of light scattering by colloidal particles while Brownian motion is the random movement of particles due to collision with other atoms or molecules.
- Brownian motion involves the collision of particles while the Tyndall effect involve light scattering
- Brownian motion is named after Botanist Robert Brown while Tyndall effect is named after John Tyndall
- Tyndall effect occurs in colloidal solutions since particles are large while Brownian motion occurs in gases and liquids.
- Tyndall effect of light scattering can be seen with naked eyes while random motion in Brownian motion can be seen under a light microscope
- Factors affecting the Tyndall effect are frequency, the wavelength of light, and density of colloidal solutions whereas those of Brownian motion are temperature and concentration.
You May Also Like: Difference between Solute and Solvent
In Conclusion
The Brownian motion explains the random movement of particles by collision with other atoms or molecules whereas the Tyndall effect explains the scattering of light by the colloidal solutions of different sizes.
More Sources and References
- https://www.thoughtco.com/definition-of-tyndall-effect-605756
- https://www.thoughtco.com/brownian-motion-definition-and-explanation-4134272
- https://en.wikipedia.org/wiki/Brownian_motion