What is the difference between phenol and benzoic acid?
Organic chemistry deals with organic compounds such as phenol and benzoic acid. These organic compounds are also known as aromatic compounds since they comprise of a planar ring system with delocalized pi-electron clouds.
The lesson provides a deeper insight into the difference between phenol and benzoic acid in a tabular form for easier understanding.
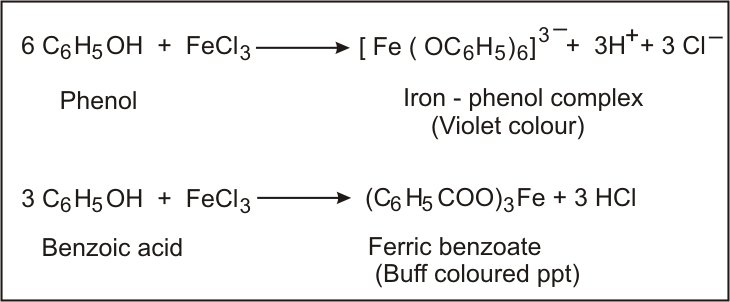
What Is Phenol?
Phenol is the simplest form of aromatic alcohol. The aromatic compound is considered to be toxic and a weak acid.
The compound has a molecular formula of C6H5OH and a molar mass of 94.11 g/mol. The molecule of phenol has benzene rings which substitute with –OH groups. One phenol molecule has one –OH group.
Characteristics of Phenol
- Has a transparent crystalline solid at room temperature and pressure.
- Produce sweet odor
- It has a melting point of 40.5 °C and a boiling point of 181.7 °C.
- Can either be in solid or liquid form
- Tend to be miscible with water due to OH group which form a hydrogen bond with water molecules
- Weak acid which dissociates partially to form phenolate anion and hydronium cation.
- Tend to be a highly toxic compound
- Use in the production of plastics, cosmetics, drugs, antiseptic and disinfectants
What Is Benzoic Acid?
Benzoic acid is the simplest form of aromatic carboxylic acid. The aromatic compound is widely used as a food preservative due to the fungistatic nature.
The aromatic compound has a molecular formula of C6H5COOH and a molar mass of 122.12 g/mol. The compound contains a benzene ring substituted with a carboxylic acid group (-COOH).
Characteristics of Benzoic Acid
- Has a white crystalline solid at room temperature and pressure
- Tend to be soluble in water
- Produce a pleasant odor
- Has a melting point of 122.41 °C and a boiling point of 249.2 °C
- Tend to decompose at a temperature beyond 370 °C
- Tend to undergo electrophilic aromatic substitution
Comparison Chart: Phenol vs Benzoic Acid
| Basic Terms | Phenol | Benzoic Acid |
| Meaning | It is the simplest alcohol | It is the simplest aromatic carboxylic acid |
| Molecular Formula | C6H5OH | C6H5COOH |
| Molecular Mass | 94.11 g/mol | 122.12 g/mol |
| Substitution | Has a –OH substituted benzene ring | Has a –COOH substituted benzene ring |
| Boiling Point | 181.7 °C | 249.2 °C |
| Melting Point | 40.5 °C | 122.41 °C |
| Water Solubility | Tend to be miscible with water | Slightly soluble in water |
| Toxicity | Toxic | Non-toxic |
| Odor | Sweet | Pleasant |
| Application | Production of plastics, cosmetics, and drugs | Preservation of food |
Core Difference between Phenol and Benzoic Acid
- Phenol is the simplest alcohol while benzoic acid is the simplest aromatic carboxylic acid.
- Phenol has a molecular formula of C6H5OH while benzoic acid has a molecular formula of C6H5COOH.
- The molar mass of phenol is about 94.11 g/mol while that of benzoic acid is about 122.12 g/mol.
- The melting point of phenol is 40.5 °C while that of benzoic acid is 122.41 °C.
- The boiling point of phenol is about 181.7 °C whereas that of benzoic acid is about 249.2 °C
- Phenol is miscible in the water while benzoic acid is slightly soluble in water
- Phenol has a –OH substituted benzene ring while benzoic acid has a –COOH substituted benzene ring
- Phenol produce sweet odor while benzoic acid produce a pleasant odor
- Phenol tend to be toxic whereas benzoic acid is less toxic
- Phenol is used in the production of plastics, drugs, and cosmetics while benzoic acid is used in the preservation of food
Read More: Difference between Acid and Base
Core Similarities between Phenol and Benzoic Acid
- Both are organic compounds
- Both are used in organic chemistry
- Both are aromatic substances
- Both undergo electrophilic aromatic substitution reactions
- Both are either colorless or white crystals at room temperature and pressure.
Comparison Video
https://youtu.be/wY7qje-73VY
Summary
The core difference between phenol and benzoic acid is that phenol is the simplest alcohol while benzoic acid is the simplest aromatic carboxylic acid. The core similarity between phenol and benzoic acid is that they are aromatic compounds.