What is the difference between Molecular motion and diffusion?
The behavior of molecules of a substance can be described by the use of either molecular motion or diffusion. These molecules occur in three states of matter such as liquid, solid, and gas.
The lesson provides the core difference between molecular motion and diffusion in a tabular form for easier understanding. Let’s find out:
What Is Molecular Motion?
Molecular motion is the movement of molecules within a substance without external influence. The movement of the particles is random and occurs within the boundary of a substance.
The movement of the particles results in the collision which causes the bouncing of the molecules. The movement of a particle is solids are quite limited since they are packed together.
The movement of molecules in gases are quite rapid and experience a lot of collision due to the available large space.
The gas-particle movement are influenced by external factors such as high temperature which triggers random movement and high collision.
What Is Diffusion?
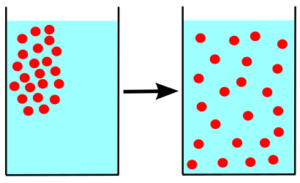
Diffusion is the movement of particles from a region of high concentration to a region of low concentration through a concentration gradient.
The movement of molecules occurs in the same solution and the factors that affect concentration gradient tend to affect diffusion as well.
When the concentration of the molecules becomes equal then the movement is terminated immediately. The molecules spread everywhere within the solution.
Comparison Chart: Molecular Motion Vs Diffusion
| Basic Terms | Molecular Motion | Diffusion |
| Meaning | Movement of molecules without any external influence | Movement of molecules from high region to low region against a concentration gradient. |
| Nature | Random movement | Against concentration gradient |
| Influences | External factors such as temperature and pressure | Factors affecting the concentration gradient |
| Medium | Solid, liquids and gases | Occur in solutions |
| Final Stage | Does not exist | When solution attains an isotonic state |
Core Difference between Molecular Motion and Diffusion
- Molecular motion is the movement of molecules without any external influence while diffusion is the movement of molecules from high to low region against the concentration gradient.
- The nature of molecules in molecular movement is random while in diffusion is against the concentration gradient
- Molecular motion occurs in the three states of matter like solid, liquid and gases while diffusion occur in solutions
- Molecular movement is influence by temperature and pressure while diffusion by factors affecting concentration gradient
- The final stage of diffusion is reached when the isotonic state is attained while the molecular movement has no final stage
Read More: Difference between Diffusion and Osmosis
Comparison Video
Summary
The core difference between molecular movement and diffusion is the molecular movement is the random movement of molecules in a substance without external factors while diffusion is the movement of molecules from a high to low region against the concentration gradient.