What is the difference between Disodium EDTA and Tetrasodium EDTA?
EDTA is an abbreviation of Ethylenediaminetetraacetic acid. It is a chelating agent that can bind with metal ions such as calcium and magnesium to result in a stable metal form.
Disodium and tetrasodium happen to be the main types of EDTA. However, these two sodium salts tend to be quite different.
The lesson provides detailed insight into the difference between disodium EDTA and tetrasodium EDTA in a tabular form.
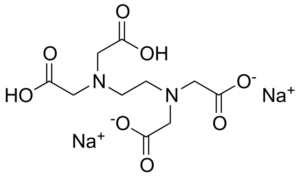
What Is Disodium EDTA?
Disodium EDTA is a salt of EDTA which contains two sodium cations. It occurs as a dry powder and it is termed as the heavy chelating agent.
The general structure of disodium EDTA shows that it has four negatively charged oxygen atoms. The two negatively charged oxygen atoms bind with two sodium cations to form a complex disodium EDTA.
The complex salt of EDTA has a molar mass of 336.2 g/mol and it is termed as a synthesized byproduct of EDTA.
The main source of sodium ions in the EDTA is sodium cyanide. The pH of the disodium EDTA solution ranges from 4 to 6.
What Is Tetrasodium EDTA?
Tetrasodium EDTA is a form of EDTA marked with four sodium cations. The four sodium cations and four negatively charged oxygen bind to form the tetrasodium EDTA compound.
The complex salt is a byproduct of EDTA synthesis with a molar mass of 380.1 g/mol. The colorless compound can either occur in dry powder or liquid form.
The compound is slightly soluble in ethanol and it has a pH that ranges from 10 to 11. It is mainly used as a water softener and preservative.
Comparison Chart: Disodium EDTA Vs Tetrasodium EDTA
| Basic Terms | Disodium EDTA | Tetrasodium EDTA |
| Meaning | It is a form of EDTA marked by two sodium cations | It is a form of EDTA marked by four sodium cations |
| Molar mass | 336.2g/mol | 380.1g/mol |
| pH Ranges | From 4 to 6 | From 10 to 11 |
| Chemical Formula | C10H14N2Na2O8 | C10H14N2Na4O8 |
Core Difference Between Disodium EDTA and Tetrasodium EDTA
- Disodium EDTA has two sodium cations while tetrasodium EDTA has four sodium cations
- The molar mass of disodium EDTA is 336.2g/mol while tetrasodium EDTA is 380.1g/mol
- The disodium EDTA pH ranges from 4 to 6 while tetrasodium EDTA ranges from 10 to 11
- The chemical formula of disodium EDTA is C10H14N2Na2O8 while that of tetrasodium EDTA is C10H14N2Na4O8
Read More: Difference between Resonance and Mesomeric Effect
Comparison Video
Summary
The core difference between disodium EDTA and tetrasodium EDTA is disodium EDTA has two sodium cations while tetrasodium EDTA has four sodium cations.