What is the difference between covalent and ionic bonds?
Chemical bonding results in the formation of a molecule or compound. Ionic and covalent bonds some of the examples used in the formation of compounds.
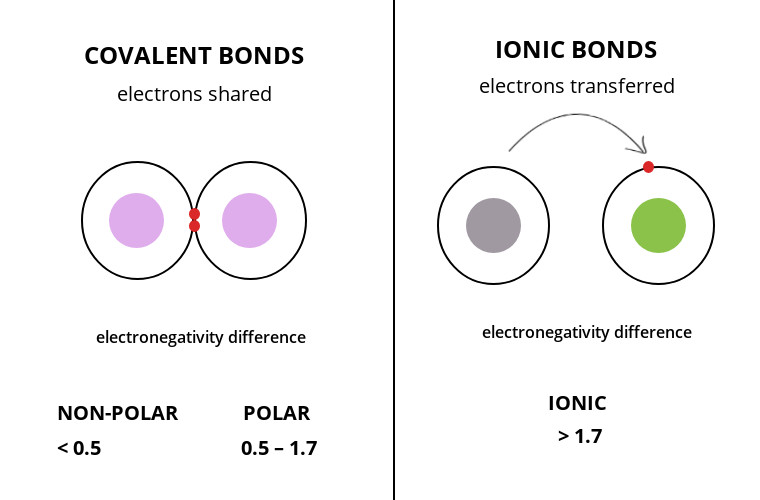
The core difference between covalent and ionic bonds in tabular form is that a covalent bond is when two species share electrons between their outer shell while the ionic bond is the electrostatic force of attraction between two opposite charged ions.
What Is Covalent Bond?
A covalent bond is a chemical bonding that occurs when non-metals combine by sharing the outermost shell electrons with each other.
Characteristics of Covalent Bond
- Have similar electronegativity values
- Exist in a gaseous or liquid state
- Grouped into polar and non-polar
- The compound dissolves in water
- Have a definite shape
What Is Ionic Bond?
An ionic bond is a chemical bonding that occurs when one atom donates a valence electron to another to attain stability.
Characteristics of Ionic Bond
- Have different electronegativity values
- Have a tendency to dissociate in water to result in ions
- Have no definite shape
- Have high melting and boiling point
- Exist in solid-state
Comparison Chart: Covalent Bonds Vs Ionic Bonds
| Basic Terms | Covalent Bonds | Ionic Bonds |
| Meaning | It is a chemical bond that occurs when atoms involve shared outer shell valence electrons | It a chemical bond that occurs when one atom donates an electron to another atom |
| Species involved | Interaction of neutral atoms | Interaction of cations and anions |
| Strength | Weak bond | Strong bond |
| Polarity | Low | High |
| Shape | Definite shape | No definite shape |
| Melting and boiling points | Low | High |
| State at room temperature | Liquid or gaseous | Solid |
| The occurrence of the chemical bond | Between non-metals | Between a metal and a non-metal |
| Bond type | Directional | Non-directional |
| Hardness | Not very hard except silicone and diamond | Tend to be quite hard due to crystalline structure |
| Speed of reaction | Comparatively low | Comparatively instantaneous |
| Solubility | Compounds not soluble in water and other polar solvents | Compounds soluble in water and other polar solvents |
| Electrical conductivity | Poor conductors | Good conductor while in a molten state |
Core Difference Between Covalent and Ionic Bonds
- Covalent bond occurs when atoms share their outer shell electrons with each other while ionic bond occurs when one atom donates an electron to another atom
- Covalent bond have low polarity while ionic bond has a high polarity
- Ionic bond has no definite shape while covalent bond has a definite shape
- Ionic bond has a high boiling point while the covalent bond has a low boiling point
- Covalent bond has a low melting point while the ionic bond has a high boiling point
- Ionic bonds exist in compounds that are solid at room temperature while covalent bonds exist in liquids or gaseous compounds at room temperature
- Ionic bonds exist between the interaction of cations and anions while covalent exist neutral atoms
- The ionic bonds are non-directional while covalent bonds are the direction
- Ionic bonds exist between a metal and non-metal while covalent bond exists between non-metals
- Covalent bonds are poor conductors whereas ionic bonds are good conductors in a molten state
- The rate of reaction of covalent bonds is comparatively low whereas that of an ionic bond is comparatively instantaneous
- Ionic bonds dissociate in water to result in ions whereas covalent bonds do not dissociate in water
- The chemical reaction between covalent bonds is molecular while that of an ionic bond is ionic in nature
- The bond angle of covalent bonds is constant whereas that of an ionic bond is variable
- Examples of ionic bond compounds are Sodium chloride and Sulfuric Acid whereas that of covalent bond compounds are Methane and Hydrochloric acid.
Read More: Difference between Covalent and Ionic Compounds
Similarities between Ionic and Covalent Bonds
- Both are strong bonds
- Both are primary bonds
- Both result in the formation of complex structures
- Both result in the formation of stable compounds
- The reaction is exothermic
- The resulting compound has a neutral charge
- Both are malleable
Comparison Video
Summary
The difference between ionic and covalent bonds is that covalent bonds are weak and the compound exists in a gaseous state whereas ionic bonds are strong and the compound exists solid state.