What is the difference between atom and molecule?
Atoms and molecules are chemistry terms that normally confuses a lot of students pursuing their studies. Some end up using them interchangeably in the wrong way.
The lesson provides the core difference between atom and molecule in tabular form for easier understanding during your revision for exams.
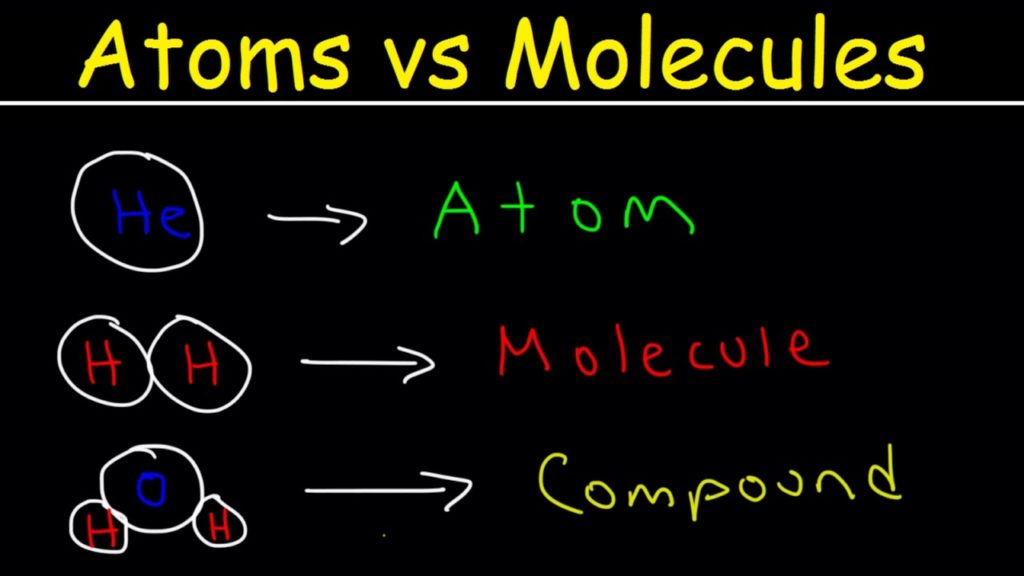
What Is An Atom?
An atom is the smallest constituent particle of an element that showcase all the chemical properties and it exists independently. Examples are H, He, Li, O, N
An atom consists of a positively charged nucleus that is surrounded by the negatively charged electrons. Electrons are negatively charged.
Both the protons and neutrons are located in the nucleus. They have equal masses but the charge tends to vary. Protons are positively charged while neutrons do not have an electric charge.
What Is A Molecule?
A molecule is the combination of one or two atoms that are held together by a chemical bond. It is also the smallest unit of matter that showcase all the chemical properties.
The chemical bond formed during an exchange of electron is known as a covalent bond. The interaction of electrons occurs when one atom loses an electron and the other gain.
Examples of molecules are H2O, CO2, NO2, and CH4 among many others. These molecules are quite visible under a magnifying microscope.
Comparison Chart
| Basic Terms | Atom | Molecule |
| Meaning | It is the smallest particle of an element that exists independently. | It is a combination of one or two atoms held together by a chemical bond. |
| Existence | Can either exist in free state or not | Exist in a free state |
| Composition | Nucleus and electrons | Two or more atoms chemically bonded together |
| Shape | Spherical | Linear, angular, rectangular, triangular |
| Visibility | Not visible with naked eyes or magnifying microscope | Visible with a magnifying microscope |
| Reactivity | Highly reactive | Relatively less reactive |
| Bond | Nuclear bond | Covalent bond |
| Stability | Unstable due to the presence of electrons | Quite stable |
| Structure | The smallest particle with the composition of an element properties | Combination of two or more atoms |
| Examples | H, He, Li, O, N | H2O, CO2, NO2, CH4 |
Core Differences
- Constituent elements of atoms are neutrons, protons, and electrons while those of molecules are a combination of two or more atoms.
- Atoms are not stable due to the presence of electrons in the outer layer while molecules attained stability easily.
- Atoms are the smallest particles with element properties while molecules are the combination of two or more atoms.
- A molecule is the combination of two or more atoms that are held together with a chemical covalent bond while an atom is the smallest particle of an element that exists independently.
- Atoms have spherical shapes while molecules have an angular, triangular, and linear shape.
- Examples of atoms are H, He, Li, O, N and that of molecules are H2O, CO2, NO2, and CH4.
You May Also Like:
- Difference between Solute and Solvent
- Difference Between Compound and Mixture
- Difference between Molecular Motion and Diffusion
Comparison Video
Summary
Both atoms and molecules are tiny units that are not visible with naked eyes. Now, what is the difference between an atom and a molecule? The core difference between atom and molecule is the presence of a nuclear bond in atoms and covalent bonds in molecules.
More Sources and References
- Matter and Elements. Khan Academy
- Molecule. Wikipedia
- Chemical Components. NCBI