what is the difference between ionic and covalent?
The majority of compounds used in chemistry can either be ionic or covalent. These types of compounds tend to differ in terms of bonding and electricity transfer.
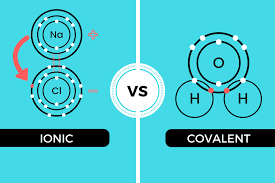
The core difference between ionic and covalent compounds in tabular form is that ionic compounds are metals with ionic bonds while covalent compounds are non-metals with covalent bonds.
What Are Ionic Compounds?
Ionic compounds refer to metallic compounds which have opposite electrical charges. This implies that the electrostatic force between each atom tends to attract each other.
The compound can either lose or gain an extra electron in the outermost shell to attain a stable electron configuration.
Cations are ionic compounds that give away an extra electron from the outer shell to have a stable state and become positively charged.
On the other hand, anions are ionic compounds that gain an extra electron in the outer shell to have a stable configuration and become negatively charged.
Therefore, the ionic bonds in ionic compounds occur as a result of anions and cations. Keep reading to note the difference between ionic solid and covalent solid.
What Are Covalent Compounds?
Covalent compounds are non-metallic compounds that result in the formation of a weak covalent bond. These compounds normally occur in the gaseous state.
The covalent solid attain stable electron configuration through the sharing of electrons in a common overlapped orbital space.
The atoms of the covalent solid come together in an orbital overlap before sharing the electrons in their outermost shell.
The resulting compound tends to be neutral since none of the atoms are charged. The overlapping tends to occur in a linear pattern.
Comparison Chart: Ionic Vs Covalent Compounds
| Basic Terms | Ionic Compounds | Covalent Compounds |
| Meaning | These are compounds with ionic bond where atoms are electrostatically attracted to each other | These are compounds where electrons are shared between atoms involved in the formation |
| Species involved | Interaction of anions and cations | Interaction of neutral atoms |
| Bonding Type | Ionic Bonds | Covalent bonds |
| Strength of the Bond | Strong | Weak |
| Metallic or Non-metallic | Metallic | Non-metallic |
| Electrical Conductivity | Good conductor in medium or liquid form | Not a good conductor |
| State of Matter | Medium to the liquid state | Gaseous state |
| Ionization | Tend to ionize in a molten solution | Do not ionize at all |
| Solubility in Organic Solvent | Do not dissolve | Tend to dissolve |
| Shape or Appearance | Have a definite shape | Do not have a definite shape |
Core Difference Between Ionic and Covalent Compounds
- Ionic compounds result in ionic bond where atoms are electrostatically attracted to each other while covalent compounds are where electrons are shared between atoms involved in the formation of covalent bonds
- The type of bonding in an ionic compound is ionic bond while in the covalent compound is a covalent bond.
- The strength of the ionic bond in an ionic compound is quite strong while the covalent bond in the covalent compound is quite weak.
- Ionic compounds are typically metallic in nature whereas covalent compounds are non-metallic in nature
- Ionic compounds are good conductors of electricity in the molten state while covalent compounds are not good conductors
- Covalent compounds occur in the gaseous state whereas ionic compound in medium to the liquid state
- Ionic compounds have definite shape while covalent compounds do not have a definite shape
- Covalent compounds dissolve in organic compounds while ionic compounds do not dissolve in organic compounds
- Ionic compounds tend to ionize in the molten state whereas covalent bonds do not ionize at all
- The interaction of cations and anions results in the formation of ionic compounds while covalent compounds are formed by the interaction of neutral atoms
Read More: Difference between Entropy and Enthalpy
Comparison Video
Summary
Therefore, ionic compounds are good conductors of electricity due to the interaction of cations and anions. On the other hand, covalent compounds do not conduct electricity due to the interaction of neutral atoms. This is the core difference between ionic compound and covalent compound brainly.