What is the difference between amylose and cellulose?
Starch is a carbohydrate which is grouped as a polysaccharide. A combination of several monosaccharide units through glycosidic bonds results in polysaccharides. These polysaccharides are polymers and monosaccharides are monomers.
The core difference between amylose and cellulose is that amylose is a stored form of polysaccharide where D-glucose molecules are linked via α-1, 4-glycosidic bond to form a linear structure while cellulose is a structural polysaccharide where D-glucose molecules are linked via β (1→4) glycosidic bonds to form a linear structure.
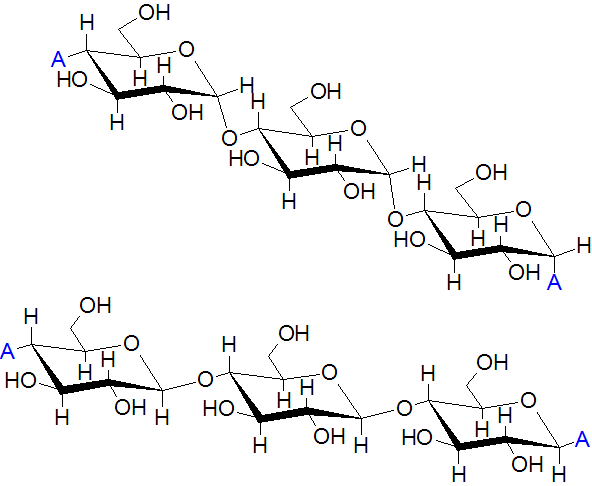
What Is Amylose?
Amylose is a linear polysaccharide structure that is formed when D-glucose molecules are joined together.
Amylose molecules are formed when several glucose units are linked together. It occurs when one carbon atom of one glucose combines with four carbon atom of another glucose molecule by a glycosidic bond.
These molecules are tightly packed together and they do not form any branches. They are not soluble in water. They are stored in plants as food while in the human digestive system convert them to maltose and glucose.
What Is Cellulose?
Cellulose is a structural polysaccharide where D-glucose are joined together. A cellulose molecule is formed when thousands of glucose molecules are joined together.
The glucose molecules are joined together by β (1→4) glycosidic bonds and they are unbranched due to the rigid structure.
The molecule formed is insoluble in water and it is quite common in green plants with the cell walls. It is used in the manufacture of biofuel, paper, and other useful byproducts.
Comparison Chart: Amylose Vs Cellulose
| Basic Terms | Amylose | Cellulose |
| Definition | It is a linear helical carbohydrate polymer made of α-D-glucose units | It is an organic polysaccharide comprising a linear chain |
| Termed as | Storage polysaccharide | Structural polysaccharide |
| Number of Monomer Units | Has 300 to several thousand of repeated glucose subunits | It has 3000 to several thousand repeated glucose subunits. |
| Structure | Linear polymer | Straight chain polymer |
| Crystalline and Amorphous Regions | Has less crystalline region | Has more crystalline region |
| Temperature of Conversion | Convert amylose crystalline to amorphous region require 60–70 °C temperature in the water | Convert cellulose crystalline into amorphous region require a temperature of 320 °C and a pressure of 25 Mpa. |
| Chemical Formula | Has no exact chemical formula | (C6H10O5)n |
| Glycoside Bonds | α(1→4) glycosidic bonds | β(1→4) linked D-glucose units |
| Significance | Plant energy storage | Structural carbohydrate |
| Iodine test or Anthrone test | Turn brown iodine solution to a blue-black color | React with anthrone in presence of sulphuric acid to form a colored compound. |
| Application | Used to form a thickening agent, water-binding agent, and gelling agent | Used to form a paper board and paper production |
| Digestion | Can be digested by salivary and pancreatic amylase into maltose | Cannot be digested by the human digestive system |
Core Difference between Amylose and Cellulose
- Amylose can be digested by the human digestive system while cellulose cannot be digested by the human digestive system.
- Amylose is used in making thickening and binding agents while cellulose for paperboard and paper production
- Amylose turn brown iodine solution to blue-black while cellulose reacts with anthrone in sulphuric acid to form a colored compound
- The significance of amylose is plant energy storage while cellulose is a structural carbohydrate
- The bond presence in amylose is α(1→4) glycosidic bonds while that of cellulose is β(1→4) linked D-glucose units
- Amylose has no exact chemical formula while that of cellulose is (C6H10O5)n
- Amylose has a linear polymer with 300 to several thousand units of glucose while cellulose is a straight-chain polymer with 3000 to several thousand units of glucose
- Amylose is a storage polysaccharide while cellulose is a structural polysaccharide
- Amylose is a linear helical carbohydrate polymer made of α-D-glucose units while cellulose is an organic polysaccharide comprising a linear chain
- Amylose has less crystalline and amorphous regions while cellulose has a more crystalline and amorphous region.
Read More: Difference between Starch, Cellulose, and Glycogen
Similarities between Amylose and Cellulose
- Both are polysaccharides
- Both are insoluble in water
- Both occur in plants
- Both have an unbranched chain
- Both form glycosidic bonds during the condensation reaction
Comparison Video
Summary
The main difference between cellulose and amylose is cellulose is a structural compound found in plants with cell walls while amylose is a carbohydrate digested by the human digestive enzymes to form maltose and glucose.