What is the difference between electrolytes and nonelectrolytes?
Compounds are classified into electrolytes and nonelectrolytes depending on the ability to produce ions and conduct electricity.
The discussion provides a detailed insight into the core difference between electrolytes and nonelectrolytes in a tabular form.
What are Electrolytes?
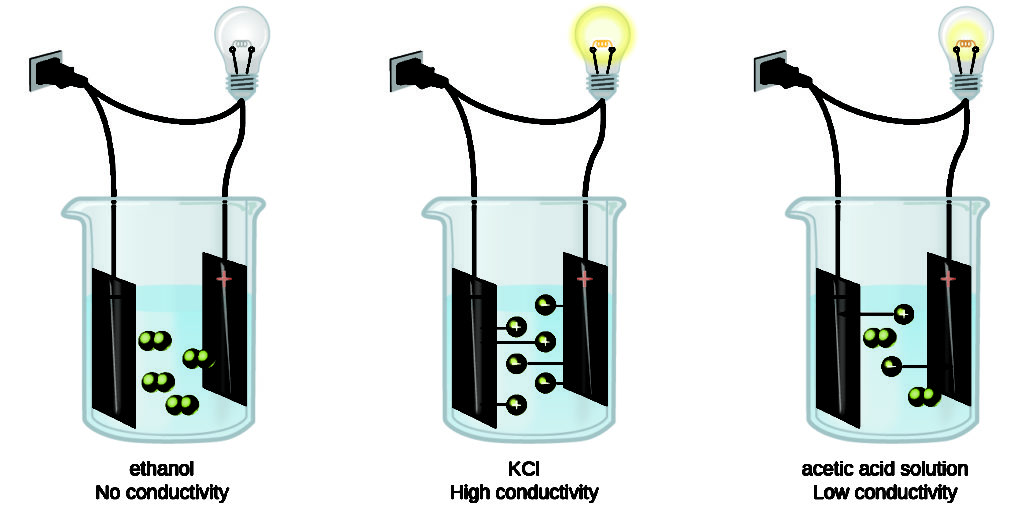
Electrolytes are compounds that can produce ions either in the molten stage or when they are dissolved in water. These free ions are responsible for conducting electricity. Carbon dioxide gas can also produce ions such as hydrogen and bicarbonate ions when dissolved in water.
Electrolytes are grouped into strong and weak electrolytes. The difference between strong and weak electrolytes is that strong electrolytes readily produce ions when they are soluble while weak electrolytes do not dissociate completely to produce ions.
What are Nonelectrolytes?
Nonelectrolytes are compounds that do not ionize at all-in solutions. Therefore, these solutions do not conduct electricity.
These compounds are held together by covalent bonds rather than ionic bonds. Examples of nonelectrolytes are sucrose, glucose, ethane, etc
Comparison Chart: Electrolytes Vs Nonelectrolytes
| Basic Terms | Electrolytes | Nonelectrolytes |
| Meaning | Are compounds that produce ions when dissolved in a solvent | Are compounds that do not produce ions when dissolved in a solvent |
| Production of Ions | Dissociate in water to produce ions | Do not dissociate in water to produce ions |
| Polarity | Polar compounds | Non-polar compounds |
| Electrical conductivity | Can conduct electricity when dissolving in a solvent | Cannot conduct electricity |
| Chemical Bonding | Ionic bonds | Covalent Bonds |
| Compounds | Acids, base, and salts | Carbon-containing compounds, fat, and sugar |
| Types | Strong and weak | Water-soluble and insoluble compounds |
| Examples | Sodium chloride and calcium phosphate | Glucose, sucrose, and ethane |
Core Difference between Electrolytes and Nonelectrolytes
- Electrolytes are compounds that produce ions when dissolving in a solvent while nonelectrolytes are compounds that cannot produce ions
- Electrolytes compounds can conduct electricity while nonelectrolytes cannot conduct electricity
- Electrolytes can be grouped as strong and weak whereas nonelectrolytes are either soluble and insoluble in water
- The chemical bonding of electrolytes is ionic bonds while that of nonelectrolytes is covalent bonds
- Electrolytes are polar compounds while nonelectrolytes are non-polar compounds
- Electrolytes dissociate in water completely to produce ions while nonelectrolytes do not dissociate in water to produce ions
- Acid, bases, and salts are examples of electrolyte compounds while sugar, fat, and carbon-containing compounds are examples of nonelectrolytes.
Read More: Difference between Natural and Synthetic Polymers
Comparison Video
Summary
The core difference between electrolytes and nonelectrolytes are electrolytes are compounds that produce ions when dissolving in a solvent whereas nonelectrolytes are compounds that cannot produce ions.