What is the difference between aldose and ketose?
Carbohydrates occur in abundance and they tend to be rich in energy. The macronutrients are quite vital for the human daily diet. They are divided into aldose and ketose sugars since they are made up of similar repetitive single units or monomers.
The lesson provides detailed insights into the difference between aldose and ketose sugars in a tabular form. Besides that, we have inserted some pictures in the discussion for easier understanding.
Read More: Difference between Reducing and Non-Reducing Sugars
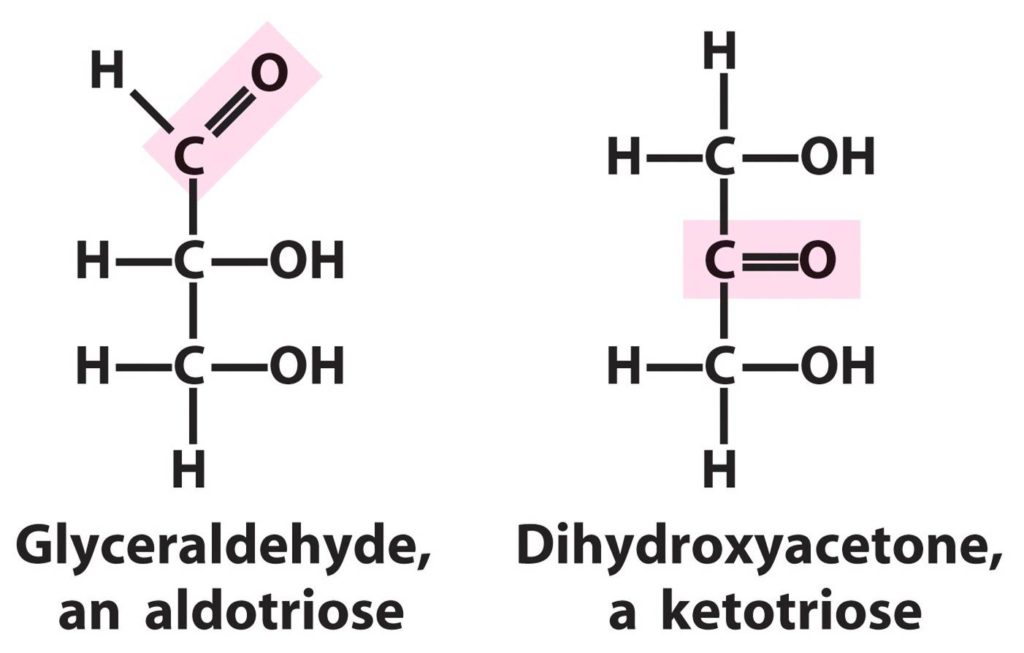
What Is Aldose?
Aldose is a monosaccharide or carbohydrate molecule that contains an aldehyde group in its structure at the end of a carbon skeleton. The chemical formula of the carbohydrate molecule is denoted as Cn(H2O)n.
The aldehyde functional group represents a carbon atom single bonded to hydrogen and a double bond to an oxygen atom. Examples of aldose sugars are glycolaldehyde, glyceraldehyde, erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, talose, and galactose.
Seliwanoff’s test happens to be the sure way to distinguish aldose from ketose. The experiment shows that aldose reacts slowly to product light pink color during the seliwanoff’s test.
What Is Ketose?
Ketose is a monosaccharide or carbohydrate molecule that contains the ketone group in its structure along with the carbon chain. The molecular formula of the carbohydrate molecule is represented as RCOR where CO represents the carbonyl group which is attached to the R group.
Ketose is a reducing agent due to a free aldehyde group or a free ketone group. A good example of ketose sugars is fructose, ribulose and xylulose, erythrulose, tagatose, sorbose, and psicose.
Ketose can easily be distinguished from aldose since it reacts with resorcinol crystalline compound to produce a dark reddish color. The presence of the carbonyl group at the end of the molecule makes ketose to be isomerized.
Comparison Chart: Aldose Vs Ketose
| Basic Terms | Aldose | Ketose |
| Meaning | It is a monosaccharide that contains an aldehyde group in its structure. | It is a monosaccharide that contains a ketone group in its structure. |
| Isomerization | Can be easily be isomerized to ketose | Can only be isomerizing if there is a carbonyl group at the end |
| Seliwanoff’s Test | React slowly to produce a light pink color | React with resorcinol to produce a dark reddish color |
| Examples | Glucose, ribose, and galactose | Fructose, erythrulose, and ribulose |
Core Difference Between Ketose and Aldose
- Aldose is a monosaccharide that has an aldehyde group in each molecule while ketose is a monosaccharide that has a ketone group in each molecule.
- Aldose structure has one carbon atom while the ketose structure has three carbon atoms
- Aldose is a pure sugar whereas ketose is an impure sugar
- The best example of aldose is glycolaldehyde while that of ketose is dihydroxyacetone
- Common examples of aldose are glycolaldehyde, glyceraldehyde, erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, talose, and galactose while that of ketose are fructose, ribulose, and xylulose.
- Aldose can easily be isomerized into ketose during isomerization response while ketose can be decomposed into aldose only if there exists a separate carbonyl group at the end of the atom.
- Seliwansoff’s test shows that aldose react slowly to produce light pink color while ketose react with resorcinol to produce a dark reddish color
- Aldose are found primarily in plants while ketose is processed in food
- Ketoses form hemiketal rings and aldoses form hemiacetal rings.
- The chemical formula of aldose is written as Cn(H2O)n while that of ketose is written as RCOR.
Read More: Difference between Glucose and Sucrose
Comparison Video
Summary
The core difference between aldose and ketose is that aldose contains an aldehyde group in each unit while ketose contains a ketone group. Both these monosaccharides can easily be distinguished through Seliwansoff’s test.